Benzene 1 4 Dicarboxylic Acid
letscamok
Sep 14, 2025 · 6 min read
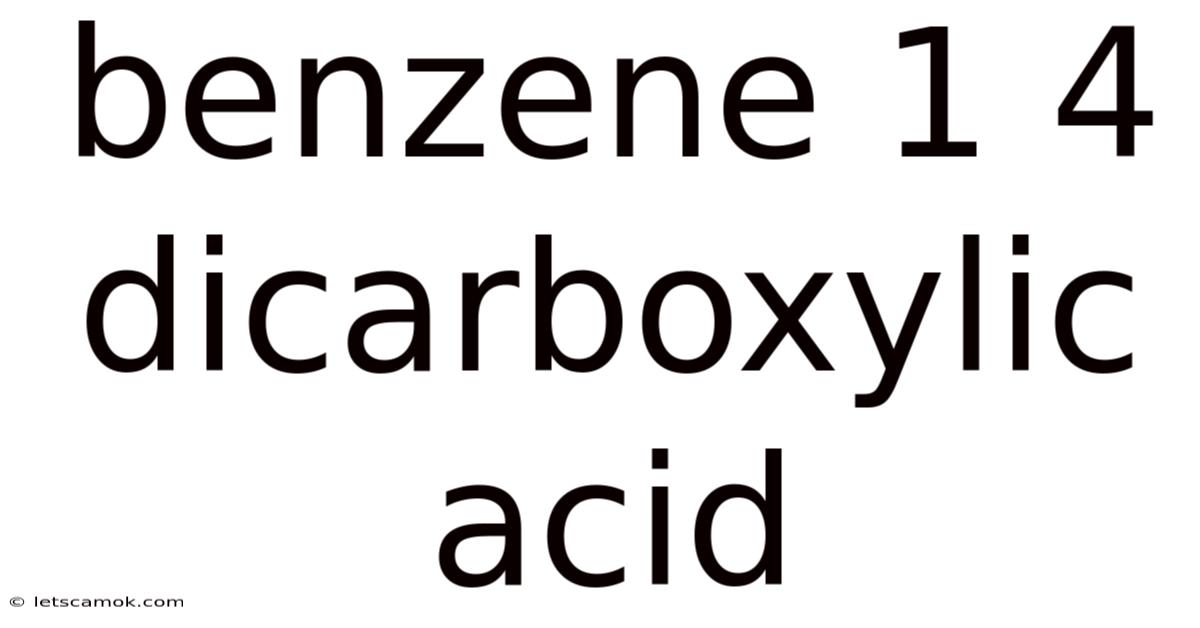
Table of Contents
Benzene-1,4-Dicarboxylic Acid: A Deep Dive into Terephthalic Acid's Properties, Production, and Applications
Benzene-1,4-dicarboxylic acid, more commonly known as terephthalic acid (TPA), is a vital building block in the chemical industry. This aromatic dicarboxylic acid holds immense significance due to its extensive use in the production of polyethylene terephthalate (PET), a ubiquitous polymer found in countless everyday products. Understanding its properties, production methods, and diverse applications is crucial for appreciating its impact on modern society. This comprehensive article delves into the world of terephthalic acid, providing a detailed overview for both students and industry professionals.
Introduction to Terephthalic Acid (TPA)
Terephthalic acid (TPA), with its chemical formula C₈H₆O₄, is a white, crystalline powder that is practically insoluble in water. Its structure features a benzene ring with two carboxyl (-COOH) groups positioned para to each other (at opposite ends of the ring). This specific arrangement of functional groups is crucial to its chemical reactivity and the properties of the polymers it forms. Its chemical name, benzene-1,4-dicarboxylic acid, clearly indicates this structural feature. The para configuration is key to the linear structure of PET, contributing to its desirable strength and properties.
Properties of Terephthalic Acid
Several key properties define terephthalic acid's suitability for various applications:
-
Melting Point: TPA boasts a high melting point, typically around 300 °C (572 °F), reflecting the strong intermolecular forces between its molecules. This high melting point contributes to its thermal stability.
-
Solubility: TPA exhibits very low solubility in water and most common organic solvents at room temperature. This low solubility influences its purification and handling processes. However, it does show some solubility in hot, concentrated alkali solutions.
-
Acidity: As a dicarboxylic acid, TPA possesses two acidic protons. These can be easily dissociated, allowing it to react with various bases and participate in esterification reactions that are fundamental to PET synthesis.
-
Crystalline Structure: The crystal structure of TPA is highly ordered, contributing to its high melting point and low solubility. This crystal structure is also important in controlling the properties of the resulting PET.
-
Reactivity: The two carboxyl groups are the main reactive sites, participating in numerous reactions such as esterification, amidation, and salt formation. These reactions are crucial for its role in polymer synthesis.
Production of Terephthalic Acid
The industrial production of terephthalic acid is a complex process, primarily involving the oxidation of p-xylene. Several steps are involved:
1. Oxidation of p-Xylene: This is the core step, where p-xylene (a derivative of benzene) is oxidized using an air or oxygen stream in the presence of a catalyst, typically a cobalt or manganese acetate. This reaction is carried out in acetic acid as a solvent, usually under pressure and at elevated temperatures (typically around 175-225°C).
2. Purification: The crude terephthalic acid obtained after oxidation requires extensive purification. This typically involves multiple steps like filtration, washing, and possibly recrystallization to remove any remaining p-xylene, byproducts, and catalyst residues. High-purity TPA is crucial for producing high-quality PET.
3. Quality Control: Rigorous quality control measures are implemented at every stage of the production process to ensure the purity and consistency of the final product. This involves various analytical techniques to determine the levels of impurities and ensure compliance with industry standards.
Importance of High Purity TPA
The purity of terephthalic acid is of paramount importance. Even small amounts of impurities can significantly impact the quality of the resulting PET, affecting its color, clarity, thermal stability, and mechanical properties. The presence of certain impurities can lead to discoloration or degradation of the polymer during processing or use.
Applications of Terephthalic Acid
The dominant application of terephthalic acid is in the production of polyethylene terephthalate (PET). PET is a widely used thermoplastic polymer with a vast array of applications, including:
-
Food and Beverage Packaging: PET is extensively used for producing bottles, containers, and films for packaging food and beverages. Its strength, clarity, and barrier properties make it ideal for these purposes.
-
Textiles: PET is used to produce polyester fibers, which are used in clothing, fabrics, and other textile applications. Its strength, resilience, and wrinkle resistance contribute to its popularity in this sector.
-
Films and Sheets: PET films are used for various applications, including packaging, photographic film, and magnetic tape. Its optical clarity and dimensional stability make it suitable for these uses.
-
Medical Applications: PET is used in various medical applications, including surgical sutures and drug delivery systems. Its biocompatibility and strength make it suitable for these purposes.
-
Other Applications: TPA is also used in the production of other polymers, such as polybutylene terephthalate (PBT) and other specialty polymers. It also finds niche applications in coatings, adhesives, and other chemical applications.
Beyond PET, TPA is a valuable intermediate in organic synthesis, finding use in the production of other chemicals and materials.
Environmental Considerations
The production of terephthalic acid, particularly the oxidation of p-xylene, involves chemical processes that raise some environmental concerns. Efforts are constantly being made to improve the sustainability of TPA production by:
-
Improving Catalyst Efficiency: Optimizing catalysts to improve conversion rates and reduce byproduct formation minimizes waste generation and resource consumption.
-
Waste Minimization: Advanced process engineering strategies are employed to minimize waste streams and recover valuable byproducts.
-
Energy Efficiency: Optimizing energy consumption in the production process reduces the overall environmental footprint.
-
Recycling: The extensive recycling of PET materials is crucial in mitigating the environmental impact of TPA use.
FAQs about Terephthalic Acid
Q1: Is terephthalic acid harmful?
A1: Pure terephthalic acid is generally considered to be of low toxicity. However, as with any chemical, appropriate handling procedures and safety precautions should always be followed. Contact with skin or eyes should be avoided.
Q2: What are the main impurities found in terephthalic acid?
A2: Common impurities can include unreacted p-xylene, other isomers of xylene, and byproducts from the oxidation reaction. The presence and concentration of these impurities can affect the quality of the final product.
Q3: Can terephthalic acid be recycled?
A3: While terephthalic acid itself is not directly recycled in a simple way, the PET polymer derived from it is widely recycled. Recycling PET reduces the demand for virgin TPA and helps conserve resources.
Q4: What are the future prospects for terephthalic acid production?
A4: The future of terephthalic acid production likely involves further advancements in catalysis, process engineering, and the incorporation of sustainable practices to improve efficiency and reduce environmental impact. Innovation in recycling and the development of biodegradable alternatives are also important areas of research.
Q5: What is the difference between terephthalic acid and isophthalic acid?
A5: Both are benzene dicarboxylic acids, but isophthalic acid has its two carboxyl groups in the meta position (1,3) rather than the para position (1,4) as in terephthalic acid. This difference in structural isomerism leads to differences in the properties of the polymers they form. Isophthalic acid is used in some polyester resins, but its applications are less widespread than those of terephthalic acid.
Conclusion
Terephthalic acid (TPA) is an indispensable chemical compound with significant industrial importance. Its dominant application in the production of PET has profoundly impacted various sectors, from packaging to textiles and beyond. While its production involves certain environmental considerations, ongoing efforts in process optimization, catalyst development, and recycling contribute to a more sustainable production cycle. Understanding the properties, production methods, and diverse applications of terephthalic acid offers valuable insights into its critical role in modern materials science and the chemical industry. Further research and development are expected to drive continued innovation and sustainability in the production and utilization of this vital compound.
Latest Posts
Latest Posts
-
Advantages And Disadvantages Trade Credit
Sep 14, 2025
-
Prince Quotes Romeo And Juliet
Sep 14, 2025
-
Area Of Complex Shapes Worksheet
Sep 14, 2025
-
How To Describe The Person
Sep 14, 2025
-
Something Messy Or Bungled Crossword
Sep 14, 2025
Related Post
Thank you for visiting our website which covers about Benzene 1 4 Dicarboxylic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.